SECCURUS™ Dry Ice Shippers
Reduce costs, streamline operations and minimize risks to frozen biologicals

Linde’s SECCURUS™ dry ice technology enables cold chain managers to reduce costs and streamline operations while minimizing risk to valuable frozen products and specimens.
The high-performance, reusable SECCURUS shipper maintains its entire storage zone at or below -70 degrees Celsius and holds temperature longer, with less dry ice than conventional dry ice shippers. Some models may last as long as 20 days.
This shipper comes conveniently preloaded with dry ice and ready to store products or specimens.
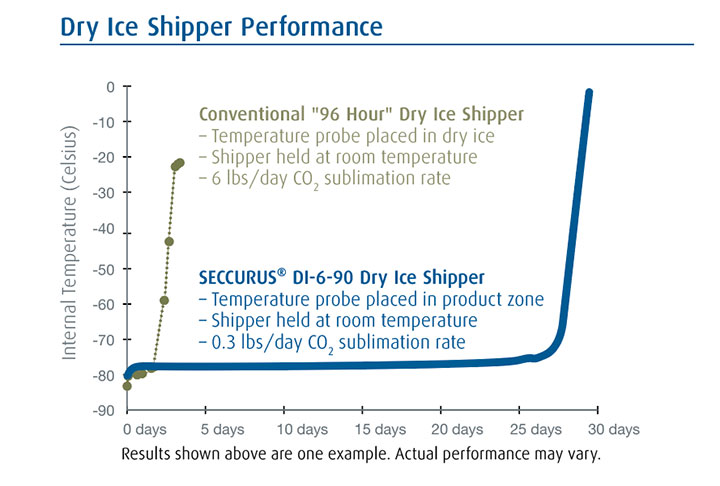
Available Models
| DI-1-90 | DI-6-90 | DI-9-152 | DI-15-216 | |
|---|---|---|---|---|
| Dry ice hold time | 7 days | 20 days | 10 days | 10 days |
| Storage zone dimensions (D x H) | 3.2 in. x 7.7 in. | 3.2 in. x 12.6 in. | 5.6 in. x 12.4 in. | 8.1 in. x 13.1 in. |
| Typical storage capacity for 2 mL vials | 20 (bag) | 80 (canes) | 200 (boxes) 300 (canes) | 500 (boxes) 600 (canes) |
| Amount of dry ice required | 2.5 lbs. | 8 lbs. | 7 lbs. | 12 lbs. |
| Complete package dimensions (L x W x H) | 10 in. x 10 in. x 16 in. | 13 in. x 13 in. x 24 in. | 13 in. x 13 in. x 24 in. | 17 in. x 17 in. x 25 in. |
| Package weight with dry ice | 10.6 lbs. | 23.0 lbs. | 24.7 lbs. | 41.4 lbs. |
All models independently validated to meet ISTA 3A, ISTA 7D and UN3373 Category B standards. All models CE marked and MDD/MDR compliant.
Applications
Biospecimens

- Pool samples for reduced number of shipments
- Frozen storage and shipping in single package
- Collect and ship samples any day of the week
- No dry ice sourcing or handling
- Reach difficult to serve locations
Cell & Gene Therapy

- Meet -70°C objective for transport and short-term storage
- Applicable to apheresis material plus cell and gene products
- Consistent duration for all shipper orientations
- Cost-efficient scale-up
Biopharmaceuticals & Vaccines
- Robust frozen preservation for 7 to 20 days
- Avoid product damage
- Flexible payload volumes
- Enable challenging supply chains
- Shipper can double as temporary frozen storage at point of administration

SECCURUS Dry Ice Shipper Advantages
Long duration - Lasts up to 20 days
Uniform payload temperature - Below -70°C for entire duration
Pre-charged with dry ice - Arrives cold and ready to use
Local frozen storage - Ship and store in same unit
Reusable - Prepaid return label included
Ship any day of the week - Can last through the weekend
Check remaining duration - Easily decide to use or replace




























